
In the coming years, it will be more and more likely to see robots in our everyday lives, whether it be at the store, in our cars, or even in our hospitals. In fact, the medical robots market is projected to reach $16.74 billion by 2023 from an estimated $6.46 billion in 2018.
The majority of growth can be attributed to technological advancements in medical robots, advantages offered by robot-assisted training in rehabilitation therapy, an increase in funding for medical robot research, and the issuance of Initial Public Offerings (IPO) by medical robot companies. Medical robots can be used in hospitals and medical centres for a variety of different purposes including administration work, diagnostic functions, physical therapy, and even surgery!
While robots may bring major benefits to the health care sector, there is one burning question: Are we ready for medical robots?
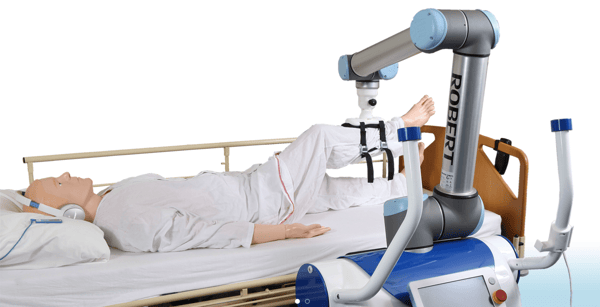
Photo Source: www.redeng.us
How Can Robots Assist in the Medical Industry?
As we all know, robots can process information much faster and much more accurately than humans. In a healthcare environment, robots can complete tasks that are normally completed by regular staff members, such as appointment booking, test result analysis, scanning vital signs and a variety of other basic functions.
RIBAs (Robot for Interactive Body Assistance) can be used to care for patients who need assistance moving, taking the physical strain off of doctors and nurses. In the near future, pharmacy automation may rely on robotic systems to dispense oral solids in retail pharmacy settings or preparing sterile IV admixtures in hospital pharmacy settings. These robots can also carry a multitude of racks, carts, or bins up to 1,000 pounds in the form of medications, laboratory specimens, or other sensitive materials – something the human body simply cannot match.
Many research companies are also experimenting with different types of robots to treat different diseases and assist with or even take over medical procedures. For example, one company is experimenting with exceptionally micro-sized robots that are smaller than a millimetre. These would be used for treating a variety of diseases and are designed to enter the body and deliver drugs at a specific location or to perform precise operations like clearing clogged arteries. Another is using a magnified 3D high-definition vision system and tiny wristed instruments that bend and rotate far greater than the human hand, operating through just a few small incisions.
Are We at Risk?
It is clear to see that these robots can bring many benefits to the health sector by completing tasks that help alleviate the strain off of healthcare workers and providing effective healthcare to patients. However, with these robots so intimately close to humans, how can we be sure they are safe? How can we ensure that a medical robot won’t malfunction during invasive surgery?
Luckily, before these robots ever make it to the market, and possibly inside of human bodies, they must first abide by a long list of safety standards. These robots must adhere to a myriad of functional safety and robotic standards, undergo vigorous risk assessments and meet a variety of technical specifications.
International Standards for Medical Electrical Equipment
While the full list of standards is too long to write out in this blog, two international standards are under development that will cover the basic safety and essential performances of Robotically Assisted Surgical Equipment (RASE) and other medical robots used for rehabilitation, assessment, compensation or alleviation, respectively.
Known as IEC DIS 80601-2-77 and IEC DIS 80601-2-78, these two particular standards will complement the main standard, IEC 60601-1. As such, these standards will be used for a more medical safety testing approach in lieu of using a combination of robotic-standards from industrial or automotive robots.
To learn the ins and outs of these standards, and the other standards governing robots and robotic devices, view our list or speak to one of our experts:



