By Bhavya H M, Sr. Executive, Business development, TÜV Rheinland India
Consistent manufacturing quality is viewed to be critical to the safety and efficacy of medical devices. According to a recent market research report, the country's medical technology sector, which has been identified as a sunrise sector with high government focus on development, is expected to hit USD 9.6 billion in 2022. The medical device industry in India is presently valued at USD 5.2 billion and is growing at 15.8% CAGR. Currently, India is counted among the top 20 in the global medical devices market and is the 4th largest medical device market in Asia after Japan, China, and South Korea, and is poised to grow to USD 50 billion by 2025 as per some industry estimates.
The medical device market is mainly dominated by imported products. The domestic companies are largely involved in manufacturing low-end products for local and as well as international consumption. We have witnessed many multinational companies establishing a local presence by acquiring established domestic companies or starting a new business. The Indian medical device market offers a great opportunity not only because of its size but also because of encouraging policies and regulations that the Government has introduced to give a boost to the medical device industry.
Medical device compliance is important to the success of every medical device manufacturer. As a manufacturer of medical devices, they bear great responsibility. The product has to guarantee benefits while meeting legal requirements. Compliance with directives or norms can be fulfilled by showing compliance with various applicable national and international standards. Testing of medical devices in accredited facilities is very important in the certification process.
The devices must be in full compliance with all applicable requirements, according to a harmonized list of standards and MDD (Medical Device Directive). It is also important to see if any other directives and standards are applicable. It is necessary to show compliance to all applicable standards and directives/norms before placing the product in the market.
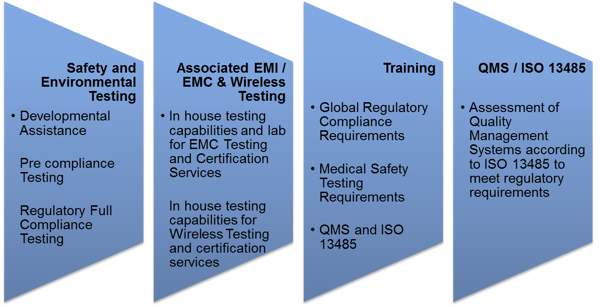
TÜV Rheinland is the one-stop-shop for all pre-compliance/ full compliance testing, inspection, assessment, training and certification requirements for medical equipment design and manufacturing companies.
Our service portfolio:
Electromagnetic Immunity and Compatibility (EMI/EMC) test:
It is important that the manufacturer identifies the right category of the product such as medical devices, in-vitro diagnostic medical devices or medicinal (drug), before looking for regulatory requirements. In many cases the difference between these categories is very small, however, there is a huge difference in the requirements. TÜV Rheinland is equipped for testing as per IEC 60601-1-2 4th edition standard. The 4th Edition of Medical EMC standard IEC 60601-1-2 is published and implemented for FDA approvals and CE marking from January 2017 and January 2019 respectively.
Reliability and Environmental Test:
Medical devices may be exposed to extreme climatic changes and mechanical stress, variations in temperature, moisture, dust, shocks or strong vibrations that impact their functionality. TÜV Rheinland labs test medical devices and their accessories for their reliability.
Electrical and Medical Safety Test:
- In house Comprehensive test facility to test and certify medical devices for various country requirements
- State-of-the-art acoustics facility to measure medical alarm requirements with the ambient noise of 18db
- Radiation test facility with lead lining for testing of X-Ray systems, Cath labs, C-Arm systems, etc.
- Test facility also includes comprehensive reliability and IP testing facility
- Medical safety and reliability test facility as per IEC, ISTA & ASTM standards
- Test facility includes medical safety as per IEC/EN/UL 60601 – 1 & -2-XX for product-specific standard series. E.g. Infant care, Imaging, Diagnosis, Therapeutic devices, etc.
- Wide variety of medical and non-medical products to be included for testing various national and international standard
Wireless Testing and Certification:
Medical devices with wireless technologies is a fast-growing market. Technologies such as Wi-Fi, Bluetooth, and ZigBee enable devices to be linked together, making the transfer of data and instructions faster and easier. Wireless medical devices must undergo extensive global testing and certification procedures to be marketed for international and country-specific standards. TÜV Rheinland helps to achieve this by conducting the testing, managing complicated international approval tasks, and interacting directly with the many certification authorities.
We offer wireless testing and international approvals for a number of wireless technology products as per the latest standards to meet Indian, European and FCC requirements. TÜV Rheinland’s global locations offer interoperability, regulatory, performance, market access, data protection (General Data Protection Regulation/GDPR) and many more test solutions for assessment and certification. Our state-of-the-art labs perform certified testing for multiple short and long-range connectivity technologies.
Our comprehensive test solutions include:
- Fully equipped test facilities for both RF Conducted Antenna Port and Radiated Spurious Emission testing
- 10-meter and 3-meter Semi-anechoic chambers, along with 3-meter Fully anechoic chamber, to perform complete testing and certification activities to meet global requirements as per NABL (ISO 17025) & A2LA (for FCC, USA & ISED, Canada)
- Testing capabilities for the frequency range of 9 kHz to 40GHz range
- FCC TCB for RF products to be placed in the USA market
- ISED TCB for RF products to be marketed in Canada
- Japan Radio & Telecommunication (JRF) testing and certification
- Notified Body under RED (Radio Equipment Directive) Directive, for European market
- LTE/GSM/CDMA/2G/3G/4G Regulatory Testing
- Turnkey certification services for Bluetooth SIG, Wi-Fi Alliance & Qi WPC (Wireless Power Consortium)
- ZigBee, LoRa & Wi-SUN Alliance’s testing services offered in-house
- SAR (Specific Absorption Rate ) testing offered locally, for both regional and global standards
- 2D & 3D Antenna performance /polar testing
Accreditations:
TÜV Rheinland India has received accreditation by;
- National Accreditation Board for Testing and Calibration Laboratories (NABL) as per ISO/IEC 17025:2017
- IECEE for CB certification for medical devices
- Notified Body under European Union
- Simultaneous national certification body for U.S.A, Germany, Japan, Argentina, Hungary, and Hong Kong
- OSHA as an NRTL and Recognized by Standards Council of Canada
- Conformity Assessment Body for Malaysia, Saudi Arabia, Brazil, and many others
- AERB to conduct Electrical Safety and Radiation Test for X-Ray devices (In house test facility)
TÜV Rheinland: Opening doors for manufactures to local and international markets
We issue the cTUVus mark, T-Mark, and Bauart mark after successful product testing against applicable standards. We can also provide test reports for technical files for European, U.S. Food and Drug Administration (FDA) approval submissions.
The range of technical services offered by TÜV Rheinland in the field of public safety, playing an important role in the medical device manufacturing industry. TÜV Rheinland looks ahead at a bright future that brings in higher standards of safety and quality regulation in this industry.



